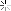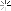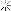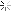Oil Analysis at 10,300 mi.
The following users liked this post:
BLKROKT (06-25-2017)
#27
Member
... I actually thought it halfway interesting that ~someone~ on this forum did a UOA, which excludes present company.
Funny how this thread lacked any useful comments until I showed up, frozen like a deer in the headlights.
ESP is something people should know about, now they do.
Funny how this thread lacked any useful comments until I showed up, frozen like a deer in the headlights.
ESP is something people should know about, now they do.

Quite happy with my oil analysis.
The following users liked this post:
Audi Junkie (08-24-2017)
#29
When I studied up on the whole extended drain issue w/Euro cars in 2001 onwards, I learned a lot, and also oil quality has changed, it's a lot better than 15 years ago. Dino oil is now better than synth of that era. Still I use synth in my turbos, but the dino oil we use now is excellent. Mobil Clean, for instance, uses a solvent-dewaxed base oil that qualifies as groupe III....a synthetic, for $15 a jug. The additive packs are the same from dino and synth, because the base oil is essentially the same "hydrocracked", the chemistry is the same....not like old "real synthetics" of PAO and ester that did eat seals.
Two quick things, Shell PZ now switched to their gas-to-liquid base oil, a true synth with not crude to refine, it's seal-neutral too. Also the new nano titanium dioxide additive that gets worked into the metal lattice from Kendall (conoco) Castrol uses it too.
Two quick things, Shell PZ now switched to their gas-to-liquid base oil, a true synth with not crude to refine, it's seal-neutral too. Also the new nano titanium dioxide additive that gets worked into the metal lattice from Kendall (conoco) Castrol uses it too.
For years antiwear additives for high-performance oils have been phosphorous compounds, particularly zinc dialkyldithiophosphate (ZDDP), that work by forming a polyphosphate film on engine parts that reduces wear. Unfortunately phosphorus is a chemical poison for automobile catalytic converters, reducing their effectiveness and life span, so industry chemists have been searching for ways to replace or reduce the use of ZDDP. It’s not a simple problem because the additive has several useful functions in addition to wear resistance.
Titanium, a protean element with applications from pigments to aerospace alloys, could step into the role of environmentally friendly additive for automotive oil, thanks to work by materials scientists from Afton Chemical Corporation (Richmond, Va.) and the National Institute of Standards and Technology (NIST).
In a recent paper, the researchers established that a titanium compound added to engine oil creates a wear-resistant nanoscale layer bound to the surface of vulnerable engine parts, making it a credible substitute for older compounds that do not coexist well with antipollution equipment.
Titanium is one candidate replacement. Mechanical tests of an organic titanium compound at Afton demonstrated that it provided superior wear resistance when added to a fully formulated engine oil, suggesting that oil chemists could use less ZDDP. Just how the titanium compound works was an open question, however. Surface analysis tests could detect titanium in the wear tracks of test surfaces but not with enough sensitivity to determine its chemical nature—and whether, for example, it was just lying there or bound to the metal surface. To resolve the issue, the researchers turned to NIST’s soft X-ray beamline at the National Synchrotron Light Source (NSLS) in Brookhaven, N.Y.
The NIST beamline instruments use low-energy (“soft”) X-rays that can be precisely tuned to specific elements to measure chemical bonds both at the surface of a sample and deeper into the bulk of the material. Powered by the NSLS, the facility is at least 10 times more sensitive than commonly available instruments. The measurements revealed that the antiwear enhancement comes from titanium chemically bound into the metal structure of the engine surface, forming a hard oxide, iron titanate. Comparing the test data to that of several possible compounds, the research team was able to identify the specific oxide. While considerably more work remains to be done, the results suggest that titanium could play an important role in future low-phosphorus lubricating oils.
Last edited by bhamg; 08-12-2017 at 12:45 AM.
The following users liked this post:
Audi Junkie (08-24-2017)